
Molecular orbital and population analysis suggest that the emission of these tetrahedral AuI complexes arises from a 3MC transition slightly perturbed by the SnCl3− fragment. It is used as a precursor to other tin compounds. Stannous chloride, solid appears as crystalline mass or flaky solid with a fatty appearance. BeF 2 and BF 3 are both two-dimensional molecules, in which the atoms lie in the same plane. The VSEPR theory therefore predicts a trigonal planar geometry for the BF 3 molecule, with a F-B-F bond angle of 120 o. O3 Molecular Geometry O3 Bond Angles O3 Shape O3 Polar or Nonpolar O3 Valence electrons In Ozone or O3, there are six valence electrons for each molecule of Oxygen. Unit cell data are known for tin (II) thiocyanate (tricltnic) and KSn (SC>4)NCS (orthorhombic) from single crystal work. Molecular Geometries and Bonding © 2009, Prentice-Hall, Inc. Feedback Bristol ChemLabS, School of Chemistry, University of Bristol, Bristol BS8 1TS, UK. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent. ∴ 2 the F2 molecule repel each other very strongly. So we can say that Carbon atom is sp hybridized. Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
#O3 MOLECULAR GEOMETRY SKIN#
It is toxic when swallowed and irritates eyes and skin when comes in contact. (c) electron geometry/molecular structure: linear. These "local structures" become something like molecular LegoTM building blocks.) Toxic by ingestion. The molecular geometry of BrF 3 is T-shaped with asymmetric charge distribution about the central atom. Show that the Raman spectrum is consistent with a trigonal-bipyamidal geometry.
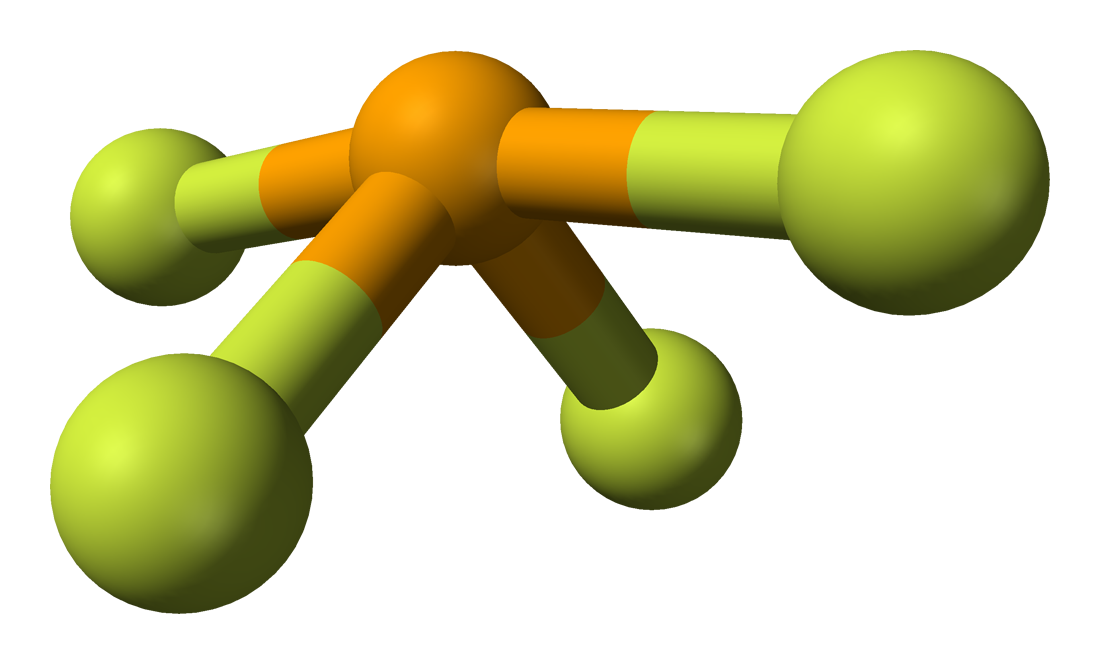
(a) XeF 4 adopts an octahedral arrangement with two lone pairs (red lines) and four bonds in the electron-pair geometry. A gas expands to fill the volume and take the shape of any container it is placed in, such as the helium in a balloon or steam formed by boiling water (Figure 1.2). It appears as a white crystalline solid which is odourless. If there are no lone electron pairs on the central atom, the electron pair and molecular geometries are the same. The SeCl4 molecule is polar because the lone pair of nonbonding electrons in the valence shell of the selenium atom interacts with the bonding pairs of electrons, causing a.
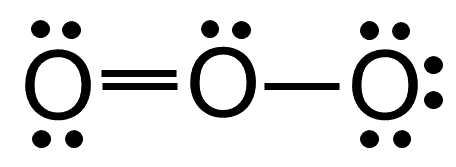
Draw the Lewis dot structures for the following. And both O atoms have sp 2 hybridization because of Oxygen has two lone pair that forms O in trigonal planar. (b) electron geometry/molecular structure: trigonal bipyramid. The elements Name Symbol Atomic number Molar mass (g mol⫺1) Name Symbol Atomic number Molar mass (g mol⫺1) Actinium Alu. In Lewis Structure formation, we have to check whether all the atoms have their least possible formal charge values. The hybridisation of Xe in XeF2 is sp3d i.e the geometry is trigonal pyramidal with 3 lone pair and 2 bond pair.


 0 kommentar(er)
0 kommentar(er)
